
ResearchManager
3.9
76
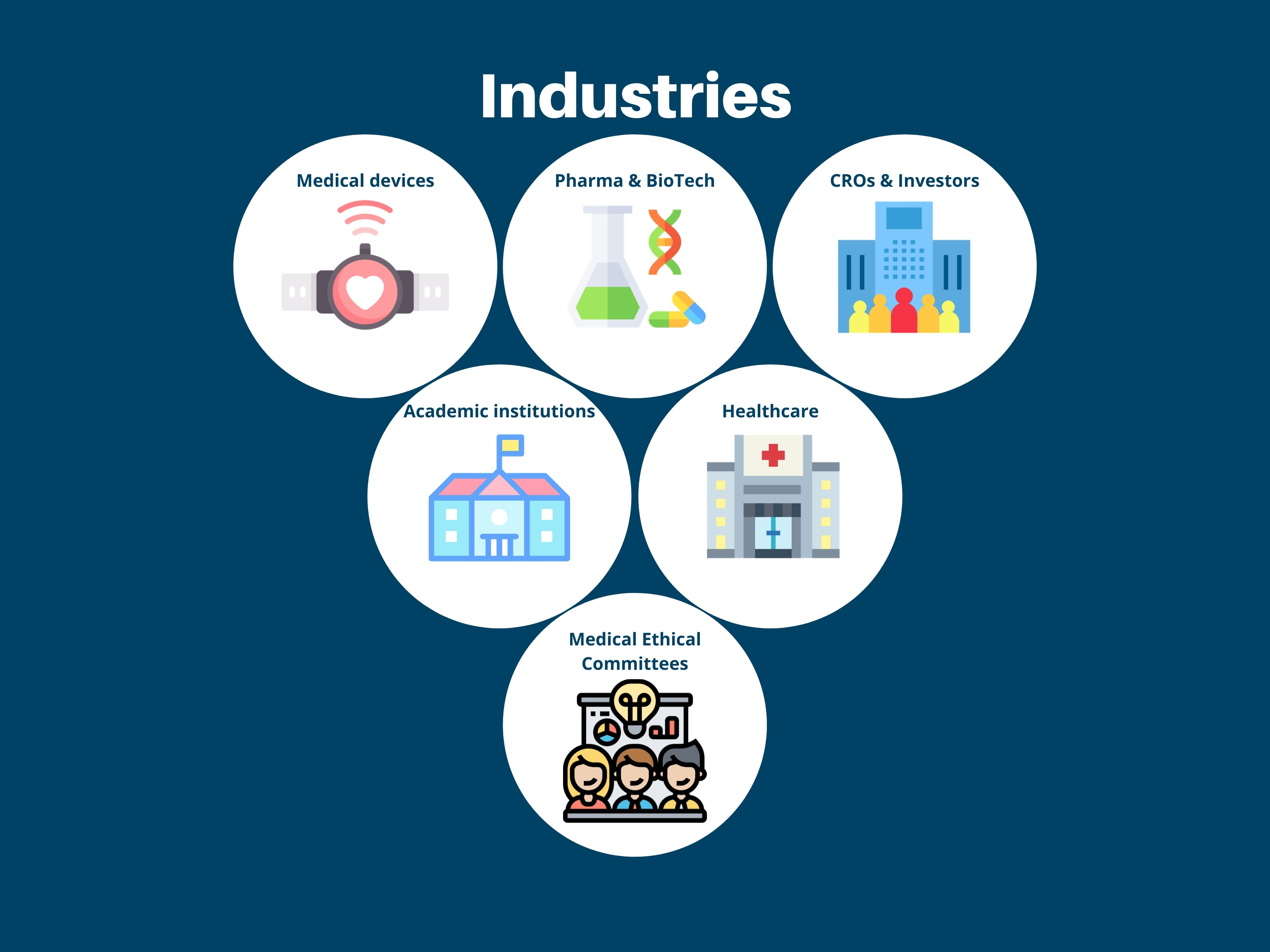
ResearchManager offers eClinical tools including CTMS, EDC, ePRO, RTSM, eConsent, and ERMS for effective management and tracking of clinical trials. They are active in 20+ countries and serve various industries including CROs, healthcare, pharma, biotech, academic institutions, medical devices, and ECs/IRBs. They are compliant with 21 CRF PART 11, HIPAA, GCP & GDPR and ISO 14155 and certified with ISO75001 & NEN7510. Customers can try the tools through a configurator, book a demonstration, or request a trial account.
Strengths
-
Customizable
Allows for tailored research projects
-
Collaborative
Enables team members to work together on projects
-
Data Visualization
Provides clear and concise visual representations of research data
Weaknesses
-
Limited Integrations
May not integrate with all necessary tools
-
Steep Learning Curve
May take time for users to fully understand and utilize all features
-
Expensive
May not be cost-effective for smaller businesses or individuals
Opportunities
- Can expand into new markets or industries
- Can add new features to stay competitive and meet customer needs
- Can form partnerships with other companies to enhance product offerings
Threats
- May face competition from other research management software providers
- May experience decreased demand during economic downturns
- May face challenges complying with changing data privacy regulations
Ask anything of ResearchManager with Workflos AI Assistant
https://my-researchmanager.com/
Apolo
Squeak squeak, I'm a cute squirrel working for Workflos and selling software.
I have extensive knowledge of our software products and am committed to
providing excellent customer service.
What are the pros and cons of the current application?
How are users evaluating the current application?
How secure is the current application?
Media










ResearchManager Plan
ResearchManager offers three pricing plans: Basic ($49/month), Pro ($99/month), and Enterprise ($199/month) with increasing features and capabilities.
Configure for a quote with this link➞ https://my-researchmanager.com/en/pricing/
69
Configure for a quote with this link➞ https://my-researchmanager.com/en/pricing/
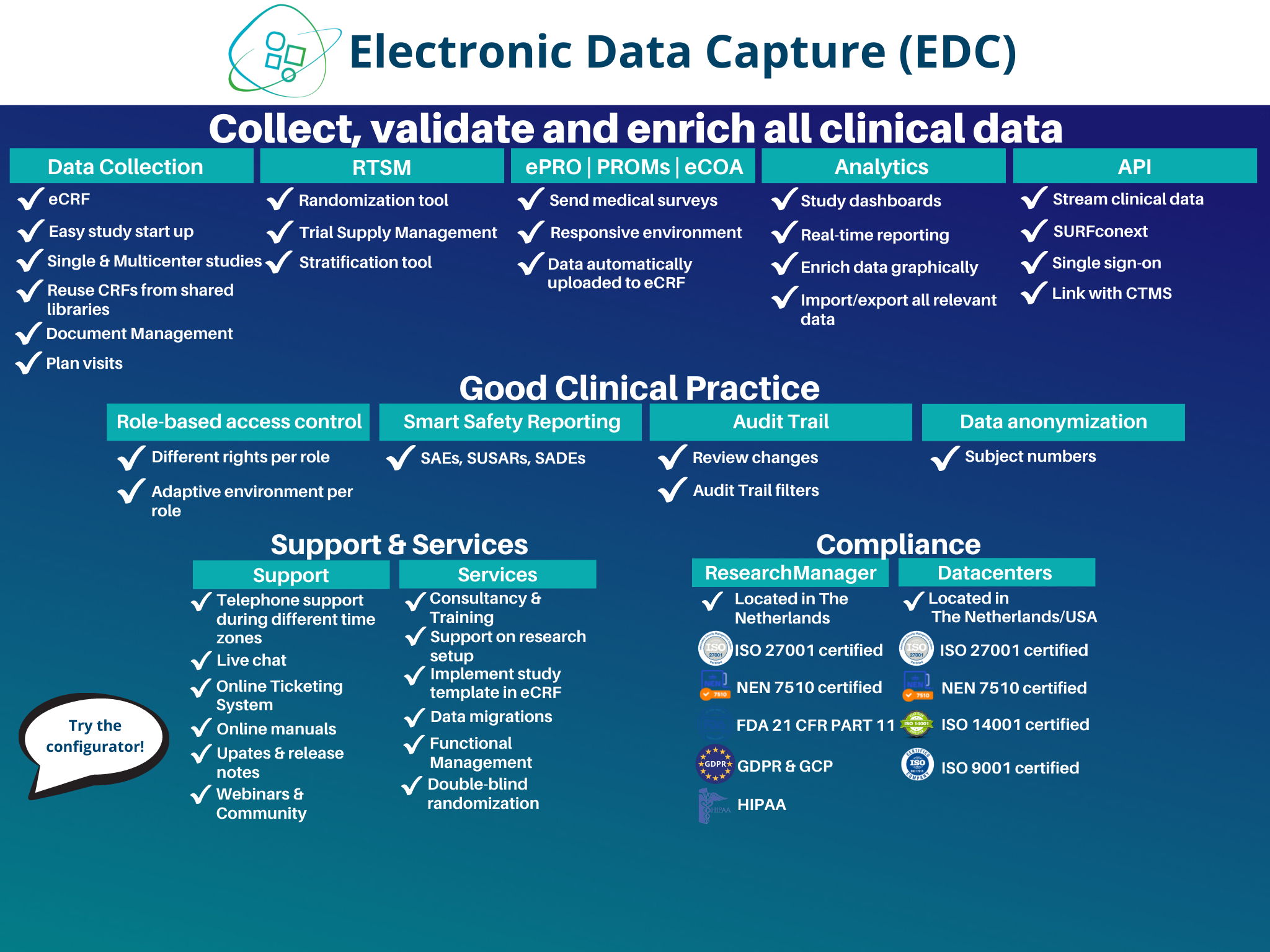
Electronic Data Capture (EDC)
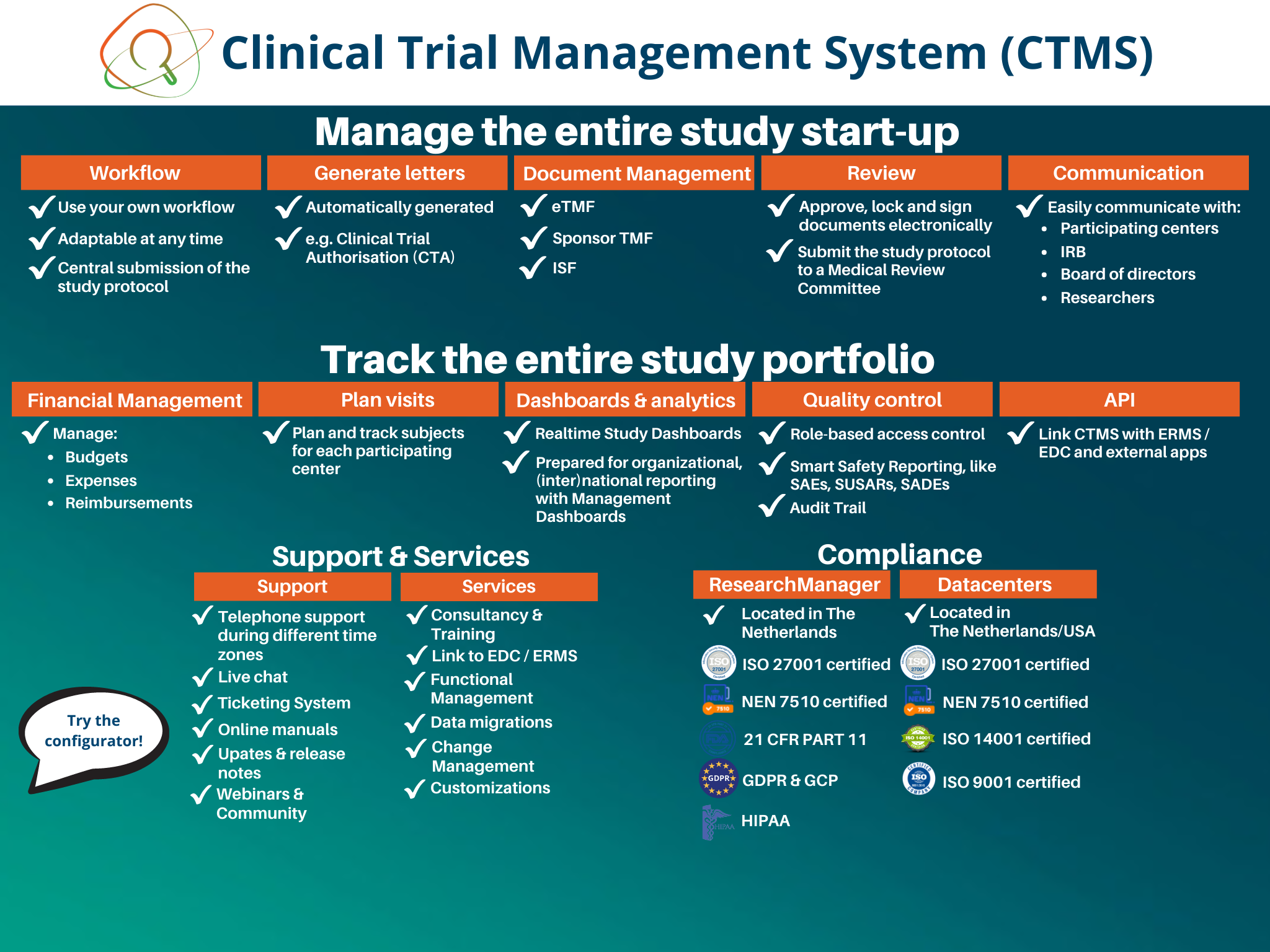
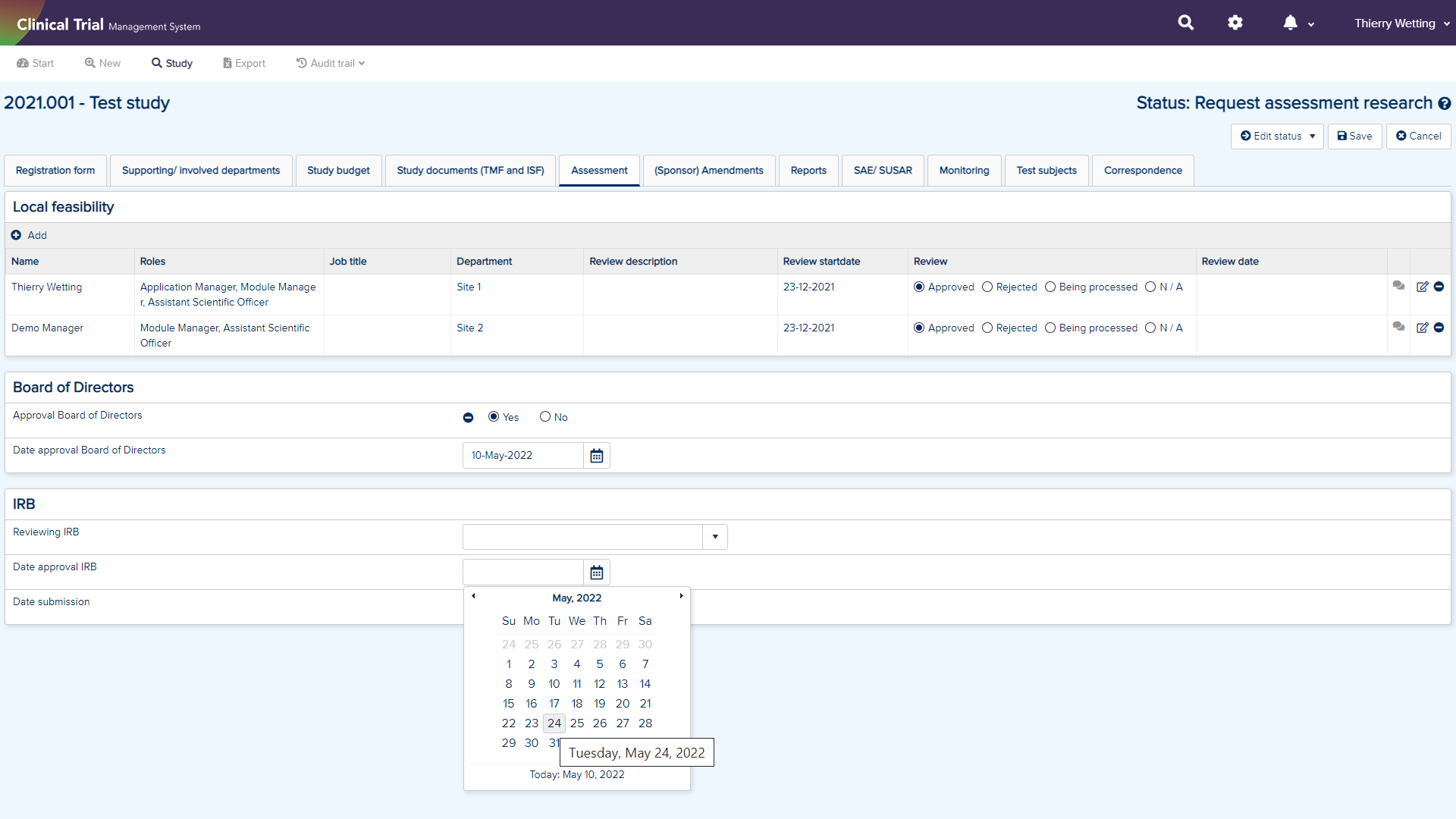
Clinical Trial Management System (CTMS)
Electronic Patient Reported Outcomes (ePRO)
Randomization and Trial Supply Management (RTSM)
Electronic informed consent (eConsent)
Ethical Review Management System (ERMS)













